FDA says no to in-home coronavirus testing – here’s what you can do instead

One of the biggest keys to successfully fighting COVID-19 is rapid, accurate testing anyone can access. In the United States, tests were not widely available until recently. And even then, you had to be showing symptoms to qualify.
But recent studies have shown this approach may have been misguided. Health officials now believe asymptomatic transmission is a primary driver of the outbreak. Tap or click here to see what other COVID-19 myths have been dispelled.
To fill in the testing gaps, several third-party startups attempted to release at-home test kits so people could confirm their symptoms. But new guidance from the FDA is putting the kibosh on these pricey kits. Here’s why.
FDA is ‘negative’ for at-home testing
Two American startups recently attempted to spearhead an effort to bring convenient COVID-19 testing to Americans. Unfortunately, new FDA rules outlined on March 21 specifically name that at-home test samples be disregarded for the sake of “safety and accuracy.”
RELATED: 5 health tests you can take online
Per the FDA’s Emergency Use Authorization guidelines, private labs will now be unable to use samples collected at home. The samples inbound from existing at-home test users are to be destroyed and discarded.
Though the reasons are vague, the FDA seems to be concerned with accuracy issues, as well as potential false negatives and positives that could skew official coronavirus statistics — which have become invaluable in our war against this disease.
One of the companies, Everlywell, is an experienced lab test maker that designs at-home kits for conditions like food sensitivity, STIs and thyroid issues.
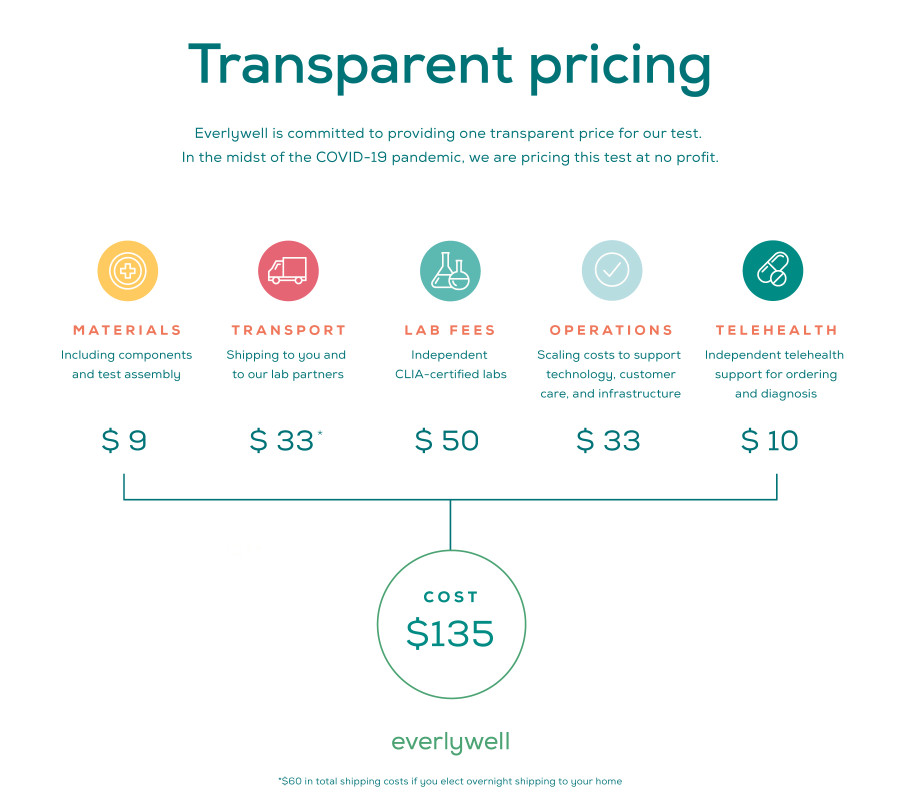
The test would have cost $135 and would have been available to order directly from Everlywell’s website. Users would have needed to answer a few questions about condition and risk factor in front of a doctor over video chat. Then, they would have submitted a throat swab and saliva sample for testing.
The Everlywell website included a graphic with a full breakdown on costs, which adds up to the total price of the kit. It’s a great way of illustrating what you’re paying for, which can remove some of the financial sting.
It also begs the question of why official test kits cost so much for those without insurance.
More options for Americans on the way
Everlywell wasn’t the only company ramping up test kit production. Scanwell, which produces app-based test kits for conditions like UTIs, is in the process of creating an at-home coronavirus test of its own.
Rather than simply provide another at-home test, Scanwell’s offering looked for something unique: the presence of antibodies. These cells are made in your body following an immune response and help your immune system identify and destroy viruses.
The presence of antibodies is usually a clear sign of a recovered infection, as well as an indicator of immunity. Some scientists hope that by extracting antibodies from recovered patients, a therapy can be developed to aggressively attack the SARS-COV2 virus.
Scanwell expects the test to ship in 6-8 weeks pending clearance under the FDA’s Emergency Use Authorization (EUA) program.
Related: Tap or click here to find out more about coronavirus scams spreading online
If you suspect you might have COVID-19 symptoms and want to test yourself, your best bet is to continue isolating yourself and to contact your physician or state health officials.
The overall availability of approved tests is still low at the moment, but the CDC has a guide on how to self-check your symptoms and what your options are if they worsen.
If your symptoms are severe, your physician or state officials may mandate you take a test at a certified medical facility.
Regardless of time frames, it’s good thing more test kits are on the way for Americans. If we know our statuses, we can perform more effective social distancing and make sure the most vulnerable among us aren’t exposed when we aren’t showing symptoms.
This battle is a marathon, not a sprint. Real results for our efforts will take weeks to show up, but if we continue toward the goal of flattening the curve of infection, we can save thousands of American lives. So stay safe and remember to wash those hands!
Tags: COVID-19, FDA, guidelines, immunity, insurance, myths, outbreak, social distancing, startups, testing, United States
